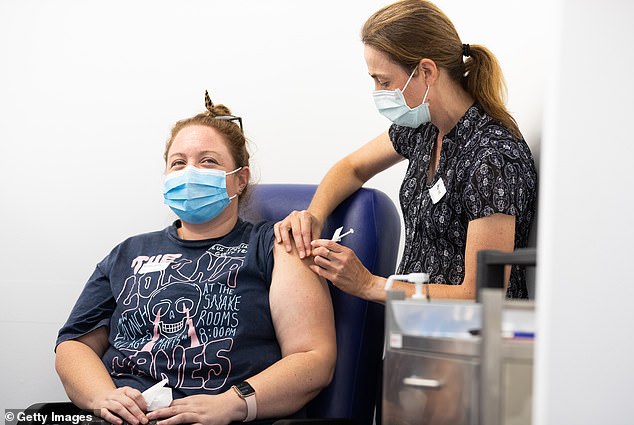
An urgent investigation has begun into the potential side effects of the AstraZeneca Covid vaccine after a man who received the jab was hositalised in Melbourne with a rare blood clotting condition.
The Therapeutic Goods Administration held talks with British regulators overnight probing whether the 44-year-old’s low blood platelets and 22 other similar cases in the UK are linked to the vaccine.
Discussions will continue on Saturday between the Australian Health Protection Principal Committee and the Australian Technical Advisory Group on Immunisation.
The medical bodies will review the concerning case and weigh the advice handed down in Friday night’s virtual meeting.

Pictured: A nurse administers the AstraZeneca COVID-19 vaccine to a patient at the Austin Hospital on March 17, 2021, in Melbourne
Australia’s medicines watchdog has begun an urgent investigation into the potential side effects of the AstraZeneca Covid vaccine after a man was hositalised with a rare blood clotting condition after receiving the jab
The patient at the centre of the investigation got the jab on March 22 and later presented to Box Hill Hospital in Melbourne suffering fever and abdominal pain.
He was found to have blood clots in his abdomen and a very low platelet count, prompting concerns from doctors.
Although there is no hard proof the AstraZeneca product can cause thrombosis syndromes in rare cases, the Australian Government has warned doctors and recipients to be on high alert.
‘People should be particularly alert to severe persistent headaches occurring four to 20 days after vaccination and which are different to the usual pattern of headaches and do not settle with over-the-counter painkillers,’ Acting Chief Medical Officer Michael Kidd said on Friday.
‘If you received the AstraZeneca vaccine and experience symptoms of persistent headaches or other worrying symptoms four to 20 days after the vaccine, you should seek medical advice.’

Australia’s leading doctor has revealed the warning signs people need to look out for after getting the AstraZeneca covid vaccine

Acting Chief Medical Officer Michael Kidd addressed concerns surrounding the vaccine on Friday
Professor Kidd said though the vaccine could cause blood clotting in people with low platelet counts he stressed that the chances of it happening were ‘very small’.
‘The serious risk disease and death from Covid-19, if we experience another severe outbreak… is far greater than the very small potential risk of a very rare clotting disorder associated with the vaccine,’ he said.
‘Cerebral venous thrombosis is a very rare disorder that has previously not been known to be associated with vaccination, however it has been noted as a complication of people who have contracted Covid-19,’ Prof Kidd said.
‘No cases of central venous sinus thrombosis have been reported in Australia to date, in the time period of concern following vaccination, which is within four to 20 days.’
Professor Kid said health authorities were taking the matter ‘very seriously’ and analysing any links between the vaccine and blood clotting.
‘This condition has presented as either a clot appearing in the brain or as thrombosis in other sites, including in the intra-abdominal venous systems,’ he said.
‘If cerebral venous sinus thrombosis or another severe thrombotic case is suspected in a patient who has received a Covid-19 vaccine, please refer them to an emergency department for further urgent assessment and haematology consultation.’

An infected nurse from Brisbane travelled to Byron Bay for a hen’s night last weekend causing the idyllic beach town to undergo tight restrictions

Pictured: A worker at CSL rolls the first batch of the AstraZeneca vaccine on March 24, 2021 in Melbourne
The Victorian Health Department warned medical professionals to be on the lookout for signs of cerebral thrombosis in patients after they had the jab.
‘As a precaution, all clinicians are being advised to refer patients who report headaches 72 hours or more after receiving an AZ Covid-19 vaccine to their nearest emergency department with a referral letter,’ the department said.
‘Hospitals will further assess the patients and may perform tests to rule out thrombosis as a precaution.’
The man’s symptoms are similar to those of prothrombotic immune thrombocytopenia in some patients who also had the vaccine.
Some countries restricted use of the AstraZeneca vaccine against Covid-19 while others have resumed inoculations, as investigations into reports of rare, and sometimes severe, blood clots continue.
Most Australians will receive the AstraZeneca jab rather than the Pfizer/BioNTech vaccine.
Earlier in the week, Germany said only people aged 60 and over should be administered the AstraZeneca vaccine due to the rare but severe occurrence of thromboembolic side effects.
The European Medicines Agency and the World Health Organization have said the benefits of the shot outweigh the risks, but are monitoring the developing situation as more cases are reported.
AstraZeneca, an Anglo-Swedish company, said earlier in March its vaccine was 76 per cent effective in preventing symptomatic coronavirus infections in a US trial, and that studies did not indicate higher risks of clotting.
The news of the Melbourne man’s symptoms comes amid critcisim over the delays of Australia’s vaccine rollout.

A generic image of AstraZeneca covid19 vaccinations inside of the Royal Exhibition Centre in Melbourne
Four million Australians were due to have their jabs by the end of March, a target missed by more than 3.3 million.
Federal opposition health spokesman Mark Butler took aim, saying the government had promised Australia would be at the front of the queue for the vaccine, but instead it had found itself in 108th place.
‘We are so far behind the rest of the world,’ he said.
‘The UK has vaccinated 60 per cent of its adult population. We’ve managed about two per cent.’
Barely a quarter of the 2.4 million vaccine doses available in the country had been administered and only a third of aged care facilities had received their first doses, he said.
‘Every promise they’ve made hasn’t been met.’
Federal Health Minister Greg Hunt on Thursday defended the Commonwealth’s vaccine rollout, saying all states and territories will receive doses in accordance with their 12-week plans.
Mr Hunt blamed a global supply issue, with the European Union blocking some vaccine shipments to Australia due to the country’s low infection rate and rising cases across Europe.